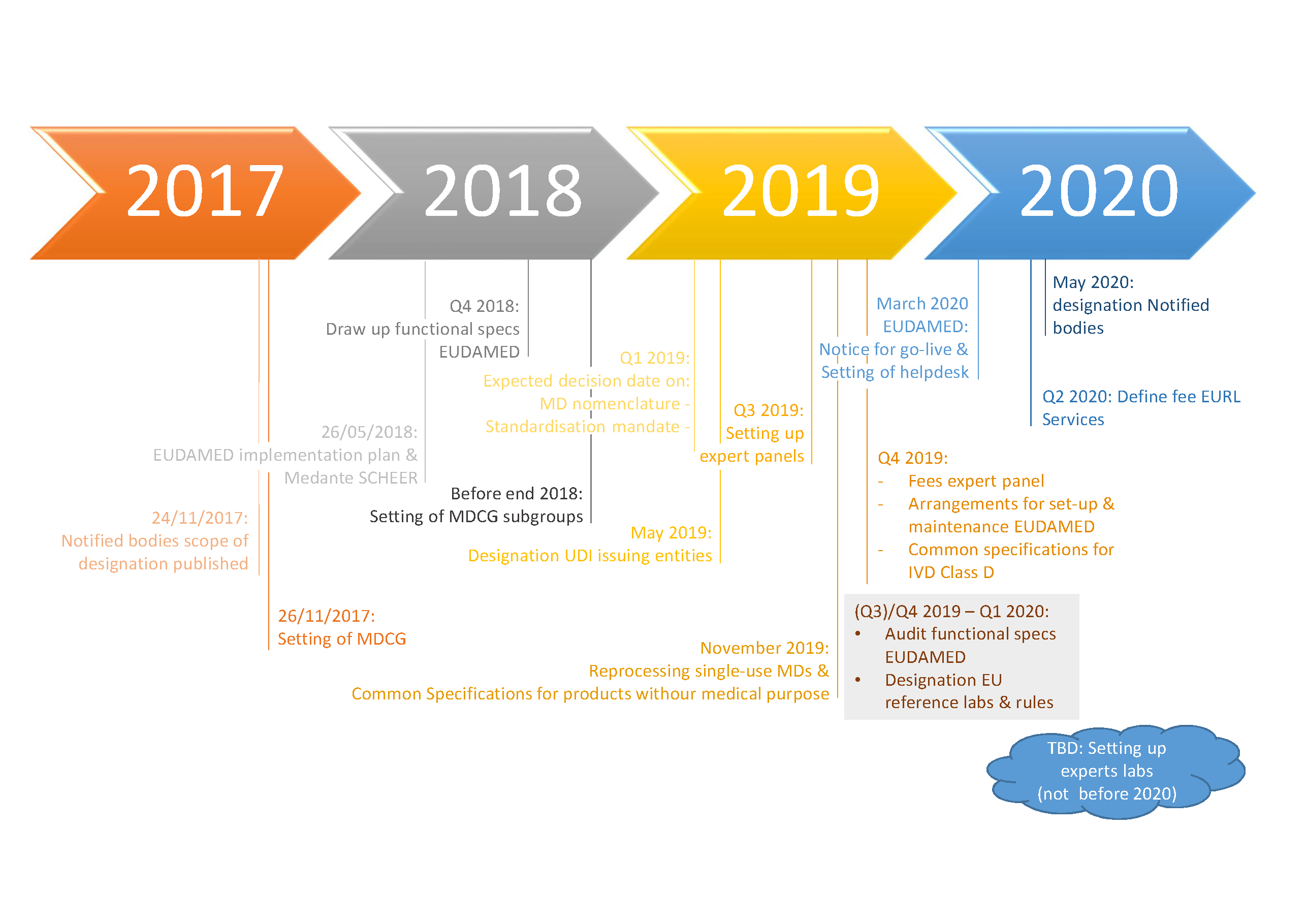
Updated EC Implementation Rolling Plan (MDR/IVDR) · MDlaw – Information platform on European medical device regulations
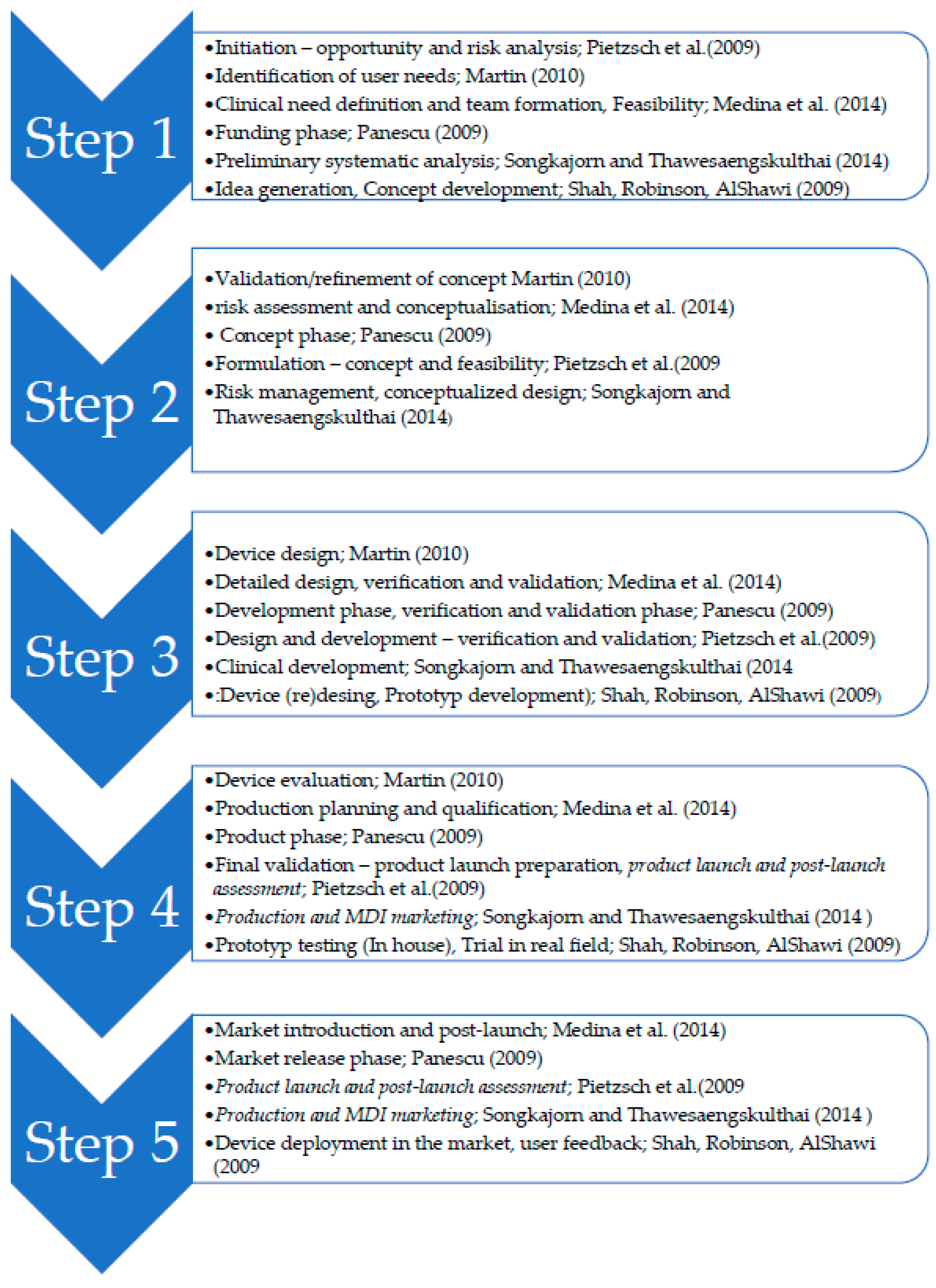
Sustainability | Free Full-Text | Complexity Stage Model of the Medical Device Development Based on Economic Evaluation—MedDee